Abstract
Introduction Initial treatment selection for older adults with AML remains challenging. Historically, clinicians and patients had to choose between intensive chemotherapy (7 + 3), low intensity therapy or best supportive care. Approval of venetoclax and a hypomethylating agent (ven/HMA, NCT02993523) for those ≥75 years or with major comorbidities has offered higher response rates and improved survival vs. low intensity therapy. Use has expanded to other groups like younger patients with high risk disease or patients with "borderline" induction fitness. Prospective comparisons have yet to complete, so we examined retrospective outcomes for older patients receiving either 7 + 3 or ven/HMA as initial therapy.
Methods We included patients from both the University of Pennsylvania ("HUP", n=187) and the Flatiron Health electronic health record derived, de-identified database with incident AML diagnosed between January 2017 and 2021, and 7 + 3 (7 days of cytarabine and 3 days of anthracycline) or ven/HMA (azacitadine or decitabine) as initial therapy (n=621). Only patients presumably eligible for 7 + 3 were included (age 60-75, estimated glomerular filtration rate > 30 mL/min, alanine transaminase, aspartate transaminase, bilirubin < 1.5x upper limit of normal (ULN), no history of systolic heart failure). Primary endpoint of overall survival (OS) had 80% power for hazard ratio (HR) 0.74 with alpha 0.05. OS was analyzed by Kaplan-Meier method and compared using log-rank test. Pre-planned sensitivity analyses included multiple imputation (MI) with inverse-probability of treatment weighting (IPTW) to address missingness and balance baseline covariates.
Results 305 patients received 7 + 3 and 503 received ven/HMA. Baseline covariates showed ven/HMA patients were older, sicker and had worse disease biology (table 1). Underscoring this imbalance in age and comorbidity, allogeneic transplant rates were 31% vs 16% for 7 + 3 and ven/HMA respectively (p<0.0005). More than 40% of initial 7 + 3 patients received second-line ven/HMA while less than 10% of ven/HMA patients received second line 7 + 3 or CPX-351.
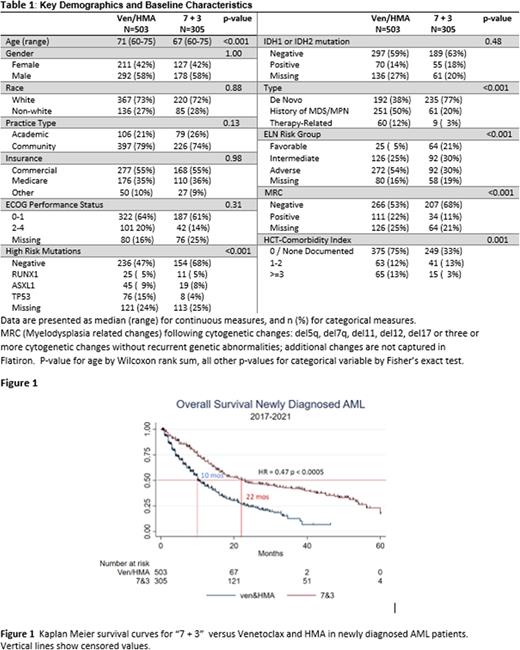
In unadjusted analysis, median OS (mOS) was 14 months for all patients and 22 months for 7 + 3 versus 10 months for ven/HMA (HR 0.52, 95% confidence interval CI 0.41-0.67, p-value <0.005, Fig 1). No subset had statistically significant higher OS with ven/HMA compared to 7 and 3; in multi-variate analysis controlling for all covariates with univariate p-value < 0.20 induction choice did not affect overall survival (limited to complete cases n=229, HR 0.72, p-value 0.21). Missing values in the HUP dataset were <5% for all baseline covariates; Flatiron dataset missing values ranged from 0-40% for covariates (e.g., sex and baseline LDH respectively). After balancing covariates and adjustment for missing values, survival remained superior in the 7+3 group (HR 0.71, p-value 0.026, 95% CI 0.53-0.94) (absolute standardized differences for all baseline variables were below 0.10).
Regarding safety, early mortality was higher for ven/HMA (13% vs 6%, p=0.003). However, rates of febrile neutropenia and culture positive infection were higher for 7 + 3. Length of stay, including any admission prior to next cycle, was longer for 7 + 3 in the HUP cohort (among patients admitted for induction: average 25 days for ven/HMA vs 34 days for 7 and 3 p=0.0012; an additional 20% of ven/HMA patients were managed entirely outpatient).
Conclusion In this large, multi-center real word study, of patients presumed to be eligible for either approach we found superior outcomes for 7 + 3. However, there was significant confounding by indication and imbalance between the groups. Adjusting for these differences, OS was still higher for 7 + 3 (p=0.026). In this real world dataset, the 7 + 3 cohort outperformed historical benchmarks (e.g., NCT02085408) in OS and early mortality, perhaps reflecting improved later lines of therapy and patient selection. Given the significant confounding by indication, uneven cross-over and greater lab-based AEs/longer length of stays for intensive chemotherapy, prospective studies (such as NCT04801797) must confirm superiority of intensive chemotherapy. Careful attention to factors predictive of response to non-7 + 3 based therapy may be as important as the characterization of side effects, quality of life and impact on allotransplant to select optimal therapies for all adult AML patients.
Disclosures
Matthews:GSK: Other: Clinical Research Training Program. Perl:Astellas, Abbvie, Daiichi Sankyo, FujiFilm, Syndax: Research Funding; Astellas, Daiichi Sankyo, AbbVie, Forma, Sumitomo Dainippon, BeatAML LLC, Loxo, LLS/Beat AML, Forma, New Link Genetics, Bayer, Biomed Valley Discoveries: Consultancy; Astellas, Daiichi Sankyo, Abbvie, Genentech, BerGenBio, Immunogen, BMS/Celgene, Actinium: Membership on an entity's Board of Directors or advisory committees. Luger:Syros, Agios, Daiichi Sankyo, Jazz Pharmaceuticals, Brystol Myers Squibb, Acceleron, Astellas, and Pfizer: Honoraria; Onconova, Celgene, Biosight, Hoffman LaRoche, and Kura: Research Funding. Gill:Novartis: Patents & Royalties, Research Funding; Immpact Bio: Honoraria; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria; Hemogenyx: Honoraria, Research Funding; Asher Bio: Research Funding; Interius: Current holder of stock options in a privately-held company, Research Funding; Carisma: Current holder of stock options in a privately-held company, Research Funding. Lai:Astellas, Jazz: Speakers Bureau; AbbVie, Agios/Servier, Daiichi-Sankyo, Jazz, Macrogenics, PDS, Pfizer, Genentech, Taiho, Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Porter:Roche: Current equity holder in publicly-traded company; Wiley: Honoraria; Bluebird Bio: Consultancy; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Tmunity Therapeutics: Patents & Royalties: anti-CD19 CART; DeCART: Consultancy; BMS: Consultancy; Novartis: Consultancy, Patents & Royalties: anti-CD19 CART, Research Funding; Genentech: Current equity holder in publicly-traded company; Elsevier: Honoraria; Mirror Biologics: Consultancy; Kadmon: Consultancy; Adecept Bio: Consultancy; Angiocrine: Consultancy; Jazz: Consultancy; Janssen: Consultancy; Gerson Lerhman Group: Consultancy; Incyte: Consultancy; Kite/Gilead: Consultancy. Carroll:Janssen Pharmaceuticals: Consultancy; Cartography Bioscences: Membership on an entity's Board of Directors or advisory committees. Babushok:Carisma Therapeutics: Current equity holder in private company; PHAR, LLC: Consultancy. Frey:Sana Biotechnology, Kite Pharma, and Syndax Pharmaceuticals: Consultancy; Novartis: Research Funding. Hexner:Tmunity Therapeutics: Research Funding; PharmaEssentia: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Samus Therapeutics, Novartis Oncology: Research Funding; American Board of Internal Medicine: Other: Member of the hematology exam committee. Stadtmauer:BMS, Celgene, Abbvie, Sorrento: Research Funding. Maillard:Genentech: Research Funding; Garuda Therapeutics: Consultancy; Regeneron: Research Funding. Pratz:AbbVie, Agios, Daiichi Sankyo, Millennium: Research Funding; AbbVie, Astellas, Boston BioMedical, BMS, Celgene, Novartis, Jazz Pharmaceuticals, and Servier.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.